From: M. Reza Ganjalikhany <ganjalikhany.gmail.com>
Date: Mon, 23 Aug 2010 13:03:15 -0700
Thank you for your email.
I'm looking for local fluctuations in a psychrophilic enzyme at different
temperatures (278,308 and 333). I found a meaningful difference at the first
experiment which was not reproduced on the iteration.
#1: (iwrap=0)
A- Heating system with weak restraints on protein in 20 ps with constant
volume from 0 K to (278,308 and 333) K
B- Equilibration at constant pressure at (278,308 and 333) K
C- Three sets of 15 ns MD simulation at (278,308 and 333)K
#2: (iwrap=1)
A- Heating system with weak restraints on protein in 40ps (constant volume)
from 0 K to (278,308 and 333) K
B- Equilibration at constant pressure at (278,308 and 333) K
C- Three sets of 15 ns MD simulation at (278,308 and 333)K
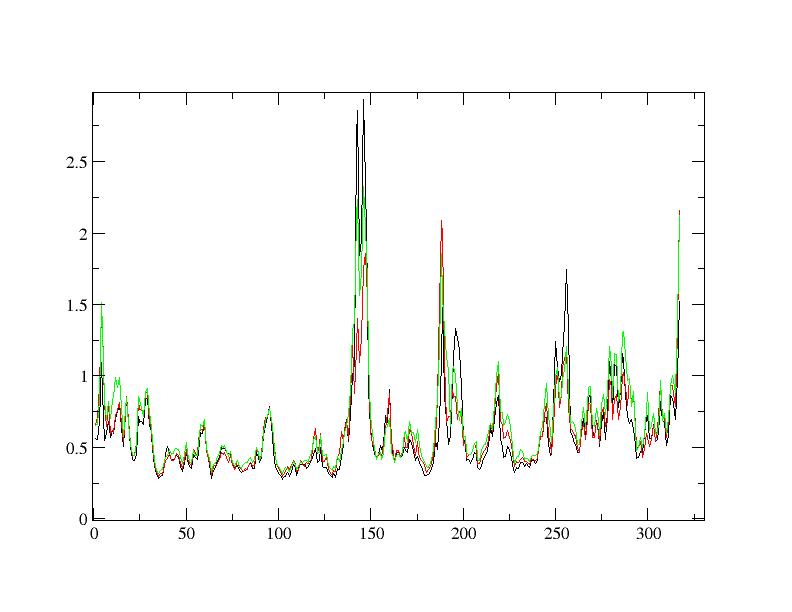
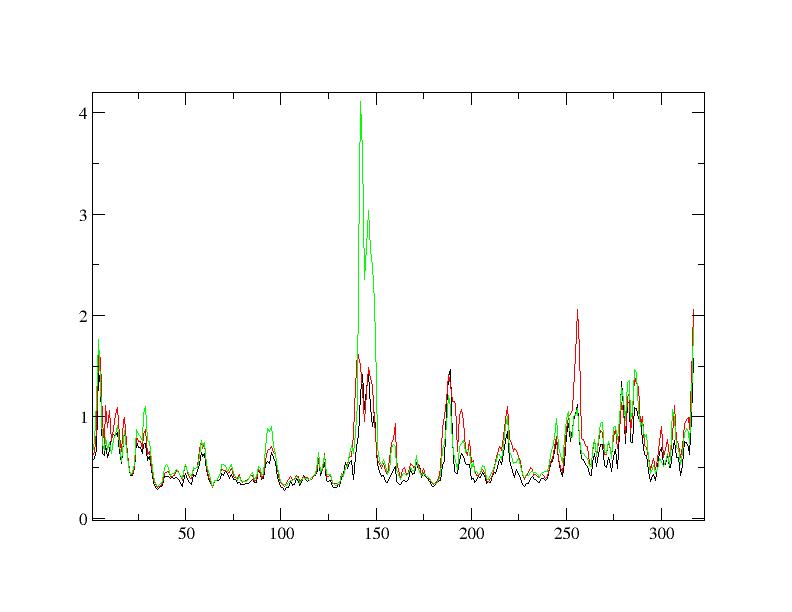
Finally I found RMSFs from experiment #1 completely different with #2 at
certain positions. On the other words, the fluctuation at certain positions
has been decreased with the temperature in #1 which was reversed in #2.
The differences in these experiments are including the heating time and the
imaging (iwrap).
RMSFs are attached.
Regards,
M.Reza
On Mon, Aug 23, 2010 at 7:26 AM, Gustavo Seabra <gustavo.seabra.gmail.com>wrote:
> Although it is true that you may be using a equilibration time that is
> too small, and that your heating is too fast, especially since you are
> using explicit solvent, the main difficulty in answering your question
> is that you also don't mention what properties you are looking at. How
> did you conclude that your results after 15ns are "completely
> different"? What properties did you measure? How do they differ?
>
> Note that, even with the exactly the same protocol, the precise
> trajectories and final structures are expected to differ between two
> different simulations.
>
> Cheers,
> Gustavo Seabra
> Professor Adjunto
> Departamento de Química Fundamental
> Universidade Federal de Pernambuco
> Fone: +55-81-2126-7417
>
>
>
> On Mon, Aug 23, 2010 at 6:01 AM, M. Reza Ganjalikhany
> <ganjalikhany.gmail.com> wrote:
> > Dear all,
> >
> > I have done two similar MD simulations with a 300 a.a. protein in
> explicit
> > solvent with different equilibration time.
> > The first equilibration time was 20ps and the second was 40 ps (from 0 K
> to
> > 300 K) and then I got completely two different results after 15ns MD
> > simulations.
> >
> > Is it normal to obtain such results? How long should be sufficient for
> such
> > system to be equilibrated?
> >
> > Any help would be greatly appreciated.
> >
> > Regards,
> > M. Reza
> > _______________________________________________
> > AMBER mailing list
> > AMBER.ambermd.org
> > http://lists.ambermd.org/mailman/listinfo/amber
> >
>
> _______________________________________________
> AMBER mailing list
> AMBER.ambermd.org
> http://lists.ambermd.org/mailman/listinfo/amber
>
_______________________________________________
AMBER mailing list
AMBER.ambermd.org
http://lists.ambermd.org/mailman/listinfo/amber
Received on Mon Aug 23 2010 - 13:30:03 PDT