Date: Mon, 3 Nov 2014 11:45:09 -0700
Hi,
After looking at the files for your system it is still unclear to me
what the problem is. You have 54 DPC molecules in an orthogonal box of
explicit water (appears to be SPC/E). The only way you could ever
center every DPC at the center of the box is if they were clustered
together.
-Dan
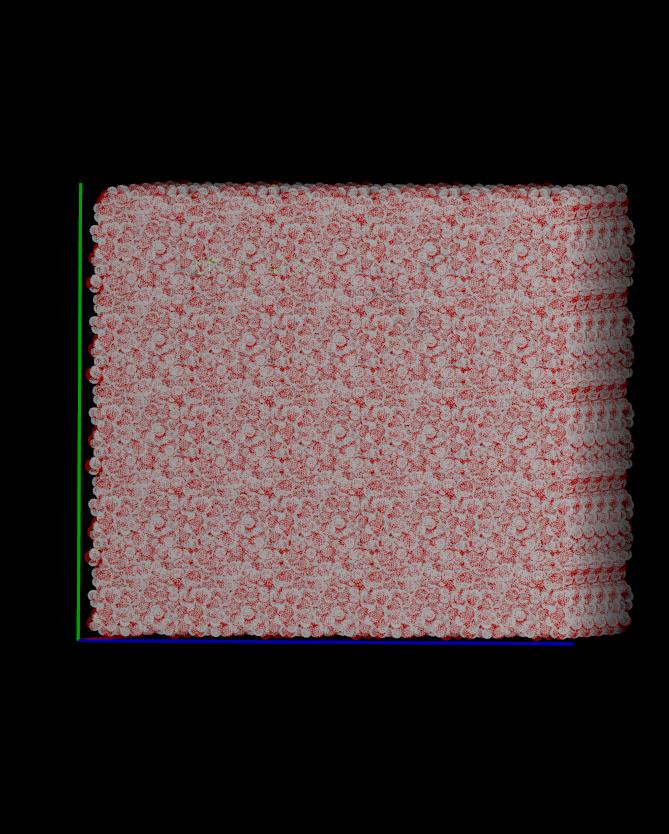
PS: There does appear to be a problem with the density in your system,
at least in the coordinates you gave me. There are gaps in your
solvent density at the edges of your box (picture attached;
A.solvent.jpg); this could lead to seemingly strange imaging results,
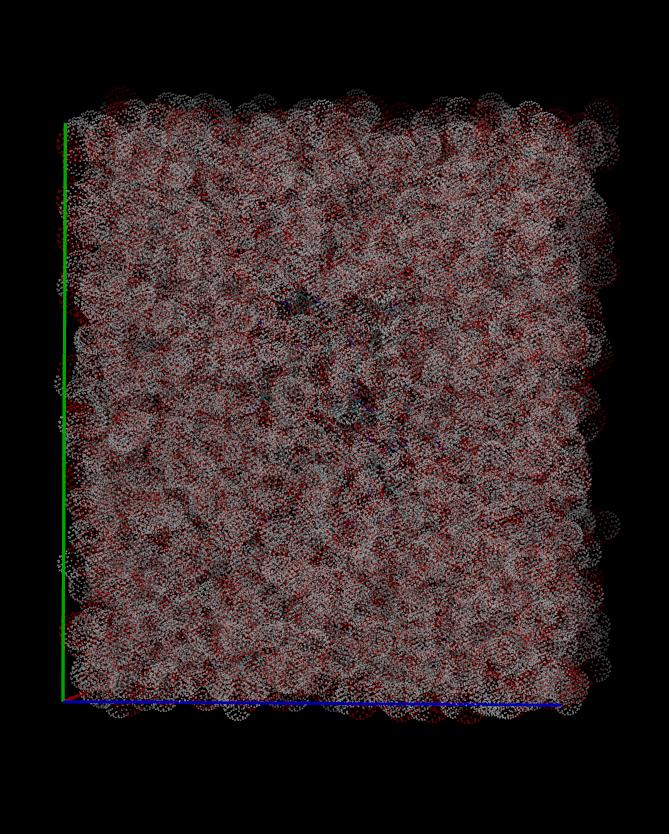
but it's just an intrinsic problem with the system density. I'm also
attaching a well-equilibrated solvent box for comparison so you can
see the difference (density.jpg).
On Thu, Oct 30, 2014 at 3:51 PM, Sylvester Tumusiime <stumusii.uno.edu> wrote:
> Hi Dr. Roe,
>
> Please let me know how i can send you the files off-list so you can have a look at them.
>
> Thanks,
>
> sylvester
> ________________________________________
> From: Daniel Roe [daniel.r.roe.gmail.com]
> Sent: Thursday, October 30, 2014 3:33 PM
> To: AMBER Mailing List
> Subject: Re: [AMBER] Imaging
>
> Hi,
>
> I think we may be hitting the limit of how much help we can provide
> without a more detailed description of your system (# molecules, their
> type, etc). If you would like to send me off-list a topology/restart
> file of your system and describe exactly what you would like to see I
> may be able to make some suggestions.
>
> -Dan
>
> On Thu, Oct 30, 2014 at 1:23 PM, Sylvester Tumusiime <stumusii.uno.edu> wrote:
>> Hi Dr. Roe,
>>
>> I implemented the suggestion you made:
>>
>>
>> What I recommend is trying something like this:
>>
>> trajin prod.netcdf
>> autoimage
>> trajout prod.imaged.nc
>>
>>
>> And it improved my trajecotry visuals: The box is not shaking violently.
>>
>>
>> However, i do still have a bunch of molecules that appear on opposite edges of the box in some instances and in other cases, they appear on the four corners of the box. They are still resistant to being centered in the middle of the box.
>>
>> Thanks,
>>
>>
>> Sylvester
>>
>>
>> ________________________________________
>> From: Sylvester Tumusiime [stumusii.uno.edu]
>> Sent: Wednesday, October 29, 2014 4:29 PM
>> To: AMBER Mailing List
>> Subject: Re: [AMBER] Imaging
>>
>> Hi Dr. Roe,
>>
>> Thank you very much for your suggestion and input. Let me do a couple of trials and make an update.
>>
>> Thanks.
>>
>> Sylvester
>> ________________________________________
>> From: Daniel Roe [daniel.r.roe.gmail.com]
>> Sent: Wednesday, October 29, 2014 3:31 PM
>> To: AMBER Mailing List
>> Subject: Re: [AMBER] Imaging
>>
>> Hi,
>>
>>
>>
>> In ptraj.in you have way too many 'image' and 'center' commands - what
>> were you trying to do? Also as I said before, your final 'center
>> :1-54' command will probably end up shifting some molecules out of the
>> box anyway.
>>
>> In ptraj1.in you are not using 'autoimage', so the results may not be
>> what you intended. The 'image' command makes no effort to keep solute
>> molecules together like 'autoimage' does.
>>
>> In ptraj2.in you also have way too many 'image' and 'center' commands,
>> but then you have an 'autoimage' command at the end which renders all
>> of those previous commands pointless anyway; i.e. instead of all the
>> 'image' and 'center' commands just use one 'autoimage' command. Was
>> there a problem with the trajectory that was output from this cpptraj
>> input?
>>
>> In ptraj3.in you have 'image' before 'center'. Also the first 'trajin'
>> command is misspelled "trajjin".
>>
>> What I recommend is trying something like this:
>>
>> trajin prod.netcdf
>> autoimage
>> trajout prod.imaged.nc
>>
>> And see if that works for you.
>>
>> -Dan
>>
>> On Wed, Oct 29, 2014 at 10:22 AM, Sylvester Tumusiime <stumusii.uno.edu> wrote:
>>> Here is one other ptraj.in file that i have used.
>>>
>>> Sylvester
>>> ________________________________________
>>> From: Daniel Roe [daniel.r.roe.gmail.com]
>>> Sent: Wednesday, October 29, 2014 10:53 AM
>>> To: AMBER Mailing List
>>> Subject: Re: [AMBER] Imaging
>>>
>>> Hi,
>>>
>>> On Wed, Oct 29, 2014 at 9:38 AM, Sylvester Tumusiime <stumusii.uno.edu> wrote:
>>>> My system consists of molecules in a solvent (different water models) with or without ions.
>>>>
>>>> After i try the different centering or imaging techniques as i mentioned, i make the following observations:
>>>>
>>>> When i look at some of the trajectories in VMD i can either see the water box shaking violently (rotating around the center) while in other simulations i can see the molecules am interested in at the edge of the box on opposite sides as you would expect if the centering had not worked.
>>>
>>> The first symptom sounds to me like RMS-fitting was performed on the
>>> trajectory. RMS-fitting a trajectory with PBC effectively invalidates
>>> the unit cell vectors since the coordinates are rotated into a new
>>> coordinate system; in other words, once you rms-fit a trajectory with
>>> PBC imaging can no longer be effectively performed. Up-to-date
>>> versions of cpptraj print a warning to this effect.
>>>
>>> What I would really need to see is all of your entire cpptraj inputs,
>>> unless the problems you are having are with your unaltered
>>> trajectories (i.e. the trajectories straight from the simulation).
>>>
>>> -Dan
>>>
>>>>
>>>>
>>>> These are the conditions i used for minimisation-equilibratiion-production
>>>>
>>>> Equilibration 1:
>>>> CZRA : equilibration
>>>> &cntrl
>>>> nstlim=100000, dt=0.002,ntx=1,irest=0,ntpr=500,ntwr=5000,ntwx=5000,
>>>> tempi=0, temp0=300.0, ntt=3, imin=0,
>>>> ntb=1, cut=10, iwrap=1,
>>>> ntc=2, ntf=2, gamma_ln = 2.0,
>>>> ntwx=200, ntwr=100, ioutfm=1,
>>>> ntr=1, restraintmask=':1-54', restraint_wt=25.0,
>>>> nmropt=1
>>>> /
>>>> &wt TYPE='TEMP0', istep1=0, istep2=100000,
>>>> value1=0, value2=300.0, /
>>>> &wt TYPE='END' /
>>>>
>>>> Equilibration 2:
>>>> CZRA : equilibration
>>>> &cntrl
>>>> nstlim=500000, dt=0.002,ntx=7,irest=1,ntpr=1000,ntwx=1000,
>>>> tempi=300.0, temp0=300.0, ntt=3, imin=0, ntwv=-1,
>>>> ntb=2, cut=8,ig=-1,ntwr=1000,
>>>> pres0 = 1.0, ntp = 1, iwrap=1,
>>>> taup = 2.0, ig=-1,
>>>> ntc=2, ntf=2, gamma_ln = 2.0,
>>>> ioutfm=1,
>>>> /
>>>> &ewald
>>>> /
>>>> ~
>>>> Minimisation 1:
>>>> minimize structure
>>>> &cntrl
>>>> imin=1,maxcyc=20000, ncyc=5000,
>>>> ntb=1, cut=8, ntwx=500, ioutfm=1,iwrap=1,
>>>> ntr=1, restraintmask=':1-54', restraint_wt=200.0,
>>>> /
>>>> &ewald
>>>> /
>>>>
>>>> Minimisation2:
>>>> minimize structure
>>>> &cntrl
>>>> imin=1,maxcyc=20000, ncyc=5000,
>>>> ntb=1, cut=8, ntwx=500, ioutfm=1,iwrap=1,
>>>> ntr=1, restraintmask=':1-54', restraint_wt=200.0,
>>>> /
>>>> &ewald
>>>> /
>>>> [stumusii.eric2 min-eqb-prod]$ cat min2.in
>>>> minimize structure
>>>> &cntrl
>>>> imin=1,maxcyc=50000, ncyc=5000,iwrap=1,
>>>> ntb=1, cut=8, ntwx=500, ioutfm=1,
>>>> /
>>>> &ewald
>>>> /
>>>>
>>>>
>>>> Production:
>>>> CZRA : equilibration
>>>> &cntrl
>>>> nstlim=100000000, dt=0.002,ntx=7,irest=1,ntpr=1000,ntwx=10000,
>>>> tempi=300.0, temp0=300.0, ntt=3, imin=0, ntwv=-1,
>>>> ntb=2, cut=8,ig=-1,ntwr=1000,
>>>> pres0 = 1.0, ntp = 1,iwrap=1,
>>>> taup = 2.0, ig=-1,
>>>> ntc=2, ntf=2, gamma_ln = 2.0,
>>>> ioutfm=1,
>>>> /
>>>> &ewald
>>>> /
>>>>
>>>>
>>>> thank you!
>>>> ________________________________________
>>>> From: Daniel Roe [daniel.r.roe.gmail.com]
>>>> Sent: Wednesday, October 29, 2014 10:20 AM
>>>> To: AMBER Mailing List
>>>> Subject: Re: [AMBER] Imaging
>>>>
>>>> Hi,
>>>>
>>>> All imaging routines ('autoimage', 'image') will correctly place atoms
>>>> inside a unit cell unless your box coordinates (used to create the
>>>> unit cell vectors) are not right for some reason, such as trying to
>>>> image after rms-fitting.
>>>>
>>>> Without a better description or example of your problem with
>>>> 'autoimage' I can't begin to help with that. However, I can tell you
>>>> that a 'center' command following an imaging command, e.g.:
>>>>
>>>>> image center :1-77562 bymask familiar
>>>>> center :1-2
>>>>
>>>> can definitely shift atoms outside the box - any action that modifies
>>>> coordinates after an imaging command can. Typically you center first,
>>>> then image (this is what 'autoimage' does internally). If you had a
>>>> 'center' command following 'autoimage' this would do the same thing.
>>>>
>>>> If you can give a more complete description of your issue I may be
>>>> able to help further.
>>>>
>>>> -Dan
>>>>
>>>> PS - As always, make sure you're using the latest version of cpptraj (14.09).
>>>>
>>>>> image center :1-77562 bymask familiar
>>>>> center :2-3
>>>>> image center :1-77562 bymask familiar
>>>>> center :3-4
>>>>> image center :1-77562 bymask familiar
>>>>> center :1-5
>>>>> image center :1-77562 bymask familiar
>>>>> center :5-6
>>>>> image center :1-77562 bymask familiar
>>>>> center :6-7
>>>>> image center :1-77562 bymask familiar
>>>>> center :7-8
>>>>> _______________________________________________
>>>>> AMBER mailing list
>>>>> AMBER.ambermd.org
>>>>> http://lists.ambermd.org/mailman/listinfo/amber
>>>>
>>>>
>>>>
>>>> --
>>>> -------------------------
>>>> Daniel R. Roe, PhD
>>>> Department of Medicinal Chemistry
>>>> University of Utah
>>>> 30 South 2000 East, Room 307
>>>> Salt Lake City, UT 84112-5820
>>>> http://home.chpc.utah.edu/~cheatham/
>>>> (801) 587-9652
>>>> (801) 585-6208 (Fax)
>>>>
>>>> _______________________________________________
>>>> AMBER mailing list
>>>> AMBER.ambermd.org
>>>> http://lists.ambermd.org/mailman/listinfo/amber
>>>>
>>>> _______________________________________________
>>>> AMBER mailing list
>>>> AMBER.ambermd.org
>>>> http://lists.ambermd.org/mailman/listinfo/amber
>>>
>>>
>>>
>>> --
>>> -------------------------
>>> Daniel R. Roe, PhD
>>> Department of Medicinal Chemistry
>>> University of Utah
>>> 30 South 2000 East, Room 307
>>> Salt Lake City, UT 84112-5820
>>> http://home.chpc.utah.edu/~cheatham/
>>> (801) 587-9652
>>> (801) 585-6208 (Fax)
>>>
>>> _______________________________________________
>>> AMBER mailing list
>>> AMBER.ambermd.org
>>> http://lists.ambermd.org/mailman/listinfo/amber
>>>
>>> _______________________________________________
>>> AMBER mailing list
>>> AMBER.ambermd.org
>>> http://lists.ambermd.org/mailman/listinfo/amber
>>>
>>
>>
>>
>> --
>> -------------------------
>> Daniel R. Roe, PhD
>> Department of Medicinal Chemistry
>> University of Utah
>> 30 South 2000 East, Room 307
>> Salt Lake City, UT 84112-5820
>> http://home.chpc.utah.edu/~cheatham/
>> (801) 587-9652
>> (801) 585-6208 (Fax)
>>
>> _______________________________________________
>> AMBER mailing list
>> AMBER.ambermd.org
>> http://lists.ambermd.org/mailman/listinfo/amber
>>
>> _______________________________________________
>> AMBER mailing list
>> AMBER.ambermd.org
>> http://lists.ambermd.org/mailman/listinfo/amber
>>
>> _______________________________________________
>> AMBER mailing list
>> AMBER.ambermd.org
>> http://lists.ambermd.org/mailman/listinfo/amber
>
>
>
> --
> -------------------------
> Daniel R. Roe, PhD
> Department of Medicinal Chemistry
> University of Utah
> 30 South 2000 East, Room 307
> Salt Lake City, UT 84112-5820
> http://home.chpc.utah.edu/~cheatham/
> (801) 587-9652
> (801) 585-6208 (Fax)
>
> _______________________________________________
> AMBER mailing list
> AMBER.ambermd.org
> http://lists.ambermd.org/mailman/listinfo/amber
>
> _______________________________________________
> AMBER mailing list
> AMBER.ambermd.org
> http://lists.ambermd.org/mailman/listinfo/amber
-- ------------------------- Daniel R. Roe, PhD Department of Medicinal Chemistry University of Utah 30 South 2000 East, Room 307 Salt Lake City, UT 84112-5820 http://home.chpc.utah.edu/~cheatham/ (801) 587-9652 (801) 585-6208 (Fax)
_______________________________________________
AMBER mailing list
AMBER.ambermd.org
http://lists.ambermd.org/mailman/listinfo/amber
(image/jpeg attachment: A.solvent.jpg)
(image/jpeg attachment: density.jpg)
