Date: Fri, 21 Apr 2017 10:58:01 -0400
Dear Dr. David Case,
Thank you for your reply. I agree that Hvib and Svib are not clearly
defined here. I learned two terms come from here:
http://www.colby.edu/chemistry/PChem/notes/NormalModesText.pdf .
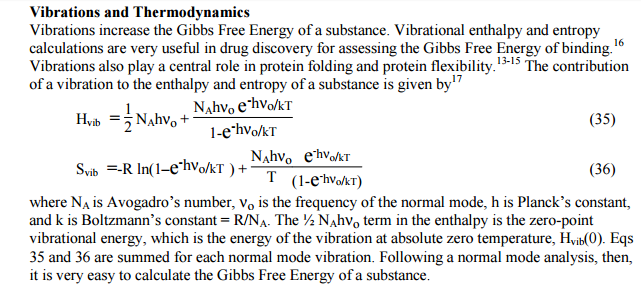
In this PChem material, vibrational frequencies are used to calculate the
contribution to Hvib and Svib. I took a snapshot below. (They are on Page
9-10 under "Vibrations and Thermodynamics" section.) I think the equations
derive from quantum theory...? Would the paragraph below clarify about Hvib
definition?
Furthermore, you mentioned that in RRHO, Evib=Hvib as vibrations don't
contribute to PV; which means this Evib refers to internal energy of the
system? I know this E, along with Cv, and S are all printed in nmode()
output. Could you explain a little bit more about the relationship between
Evib and S? Are the S and E in nmode() program calculated from the two
equations above?
Thanks a lot!
Guqin
On Thu, Apr 20, 2017 at 9:48 PM, David Case <david.case.rutgers.edu> wrote:
> On Thu, Apr 20, 2017, Guqin Shi wrote:
> >
> > As I am reviewing NMA basics, I noticed that in theory, low frequency
> > vibration (lower than 500 cm-1) could have non-negligible contribution to
> > enthalpy. Cases include torsional vibration, ring bending vibration, etc.
>
> I'm lost here: in the rigid-rotor harmonic oscillator approximation (which
> is what makes sense for normal mode thermochemistry) Evib = Hvib, as
> vibrations don't contribute to pV (we are also in the ideal gas limit.)
> Evib is printed for each frequency in the nmode() output.
>
> If I am missing something here, you would need to provide a precise
> definition
> of what you mean by "Hvib".
>
> Basically, in the MM/PBSA model, the internal energies are made via
> averaging
> potential energies over snapshots; pV terms in the gas phase assume ideal
> gas
> behavior; pV terms in solution are ignored; configurational entropy terms
> are estimated via the RRHO approximation. You don't want to try to make
> use of Evib since that would amount to double-counting the internal energy
> part.
>
> ...hope this helps...dac
>
>
> _______________________________________________
> AMBER mailing list
> AMBER.ambermd.org
> http://lists.ambermd.org/mailman/listinfo/amber
>
-- Guqin SHI PhD Candidate in Medicinal Chemistry and Pharmacognosy College of Pharmacy The Ohio State University Columbus, OH, 43210 https://www.linkedin.com/in/guqin-shi-029bb634
_______________________________________________
AMBER mailing list
AMBER.ambermd.org
http://lists.ambermd.org/mailman/listinfo/amber
(image/png attachment: 1.png)
